[ Instrument Network Instrument R & D ] Recently, Zhou Gang (co-author), assistant researcher of the Titanium Alloy Research Department of the Institute of Metal Research, Chinese Academy of Sciences, Wang Yan, associate researcher, and Li Dongsheng (corresponding author) and Ph.D. Song (co-author) One work) and Dr. Lu Ning (co-worker) of the University of Michigan, etc., used high-resolution in situ transmission electron microscopy and molecular dynamics simulation methods to reveal the formation mechanism of two five-fold twins at the atomic scale. Related research results were published online in Science on January 3, 2020.
As an important twin structure, pentad twins are widely used in the fields of crystal growth, biomedicine, optics and catalysis. For example, the lattice distortion introduced by the five-fold twin structure can increase the Young's modulus of the nanowires; the five-fold twin copper nanowires show excellent catalytic performance in the process of reducing carbon dioxide to produce methanol. Although G. Rose discovered the quintet twins in gold in 1831, researchers have discovered the quintet twins structure in nearly a hundred materials and conducted a lot of basic and applied research. Direct observation of the formation process, the formation mechanism is still inconclusive.
Twins refer to the fact that two crystals (or two parts of a crystal) form a mirror-symmetrical orientation relationship along a common crystal plane (that is, a specific orientation relationship). These two crystals are called "twin crystals." This common crystal plane It is called twin facet.
During the growth and preparation of crystals, the crystals will co-exist along some symmetrical operation, forming twins. The two parts of the crystal separated by the twin interface intersect with each other in a specific orientation relationship, thereby forming a new additional symmetrical element. Such as a reflection plane, a rotation axis, or a center of symmetry. But these symmetrical operations must be independent and cannot be related to any symmetrical operation in the space group to which the crystal structure belongs. At the same time, these newly added symmetry operations must also be crystallographically permitted. For the monoclinic system, its symmetry is characterized by the existence of a secondary rotation axis in the b-axis direction or reflecting the symmetry plane. The reflection plane is independent, and the reflection symmetry operation further associates the two parts of the crystal to obtain twins.
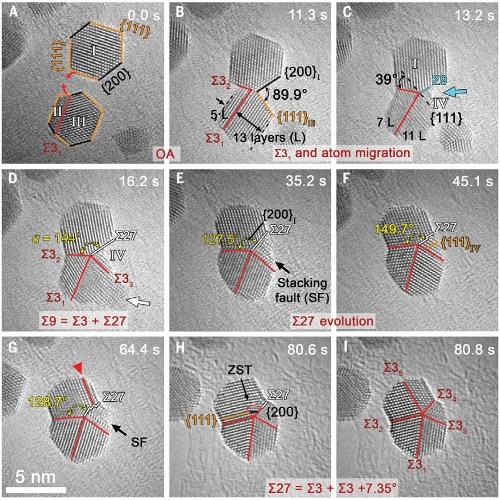
The researchers found that during the aggregate growth of ~ 3nm Au, Pt, and Pd nanoparticles, the nanoparticles can form the initial two twin interfaces through orientation attachment (OA) between the particles, and then pass through Atomic surface diffusion and formation and decomposition of high-energy grain boundaries (mechanism 1) or slippage of incomplete dislocations (mechanism 2) form a five-fold twin structure. The two formation mechanisms mainly depend on the surface structure formed after the particles are oriented and adhered. If the included angle of the concave surface is close to 90 ° after orientation adhesion, it is mechanism 1. If the included angle of the concave surface is close to 150 °, it is mechanism 2. The specific formation process is as follows:
Mechanism 1: Through orientation adhesion, atomic surface diffusion, and subsequent formation and decomposition of high-energy grain boundaries. First, the initial two Σ3 twin interfaces and a ~ 90 ° concave surface are formed through the particle orientation adhesion process; this concave surface with a larger curvature will cause surface atoms to diffuse there and form a third Σ3 twin boundary and Σ27 high-energy grain boundary; finally through the nucleation and growth of zero-strain twins near the twin poles, Σ27 decomposes into two other Σ3 twin boundaries and forms a five-fold twin structure. This mechanism can form a more symmetrical five-fold twin structure.
Mechanism 2: Slip or grain boundary decomposition by orientation adhesion and incomplete dislocations. When the included angle of the concave surface formed during the orientation adhesion process is ~ 150 °, the formation of quintwinning twins can be achieved by slipping of incomplete dislocations in the surface atoms or decomposition of the Σ9 grain boundary. Continued slip of incomplete dislocations can promote the twin interface to migrate into the grain, but also with the increase of lattice strain energy, so this mechanism mainly forms an asymmetric five-fold twin structure. In the subsequent growth process, the evolution of the pentad twin symmetry can be achieved by growing up with other nanoparticles.
Nano particles, also known as nano dust, nano dust, refers to micro particles on the order of nanometers. It is defined as particles smaller than 100 nanometers in at least one dimension. Semiconductor nanoparticles smaller than 10 nanometers are also called quantum dots because of their quantized electronic energy levels.
Nano-particles are artificially made micro-particles with a size not exceeding 100 nanometers. Its morphology may be latex, polymer, ceramic particles, metal particles and carbon particles. Nanoparticles are increasingly used in medicine, sunscreen cosmetics, and the like.
Nanoparticles are able to penetrate into membrane cells and spread along synapses, blood vessels and lymph vessels of nerve cells. At the same time, nanoparticles are selectively accumulated in different cells and certain cell structures. The strong permeability of nanoparticles not only provides effectiveness for the use of drugs, but also poses a potential threat to human health. But so far, little research has been done on the harm of nanoparticles to human health.
Air Grip Union,Air Inflatable Tube,Seal-O-Grip Air Union,Seal O Grip Union
Hebei Huayu Special Rubber Co.,Ltd , https://www.api7khose.com